SkyDance is comprised of industry veterans with collective backgrounds that span leading medical device, enterprise software, and vascular access organizations including St. Jude, C.R. Bard, Med-Comp, Medibuy.com, B. Braun, Vascular Pathways, Covidien, Lumitec, and Kimberly Clark.
Please reach out to us for more information…
561.573.5360
info@skydancevascular.com
1104 Nassau Street, Suite 107, Delray Beach, FL 33483

SkyDance Vascular, Inc. announced the U.S. Food and Drug Administration (FDA) 510(k) clearance of the Osprey Peripheral IV Catheter System (OspreyIV), a revolution in peripheral vascular access. The Company also announced additional patent awards from the United States Patent and Trademark Office (USPTO), strengthening the Company’s claim that its products are designed to reduce the number of known complications associated with the traditional PIVC’s. The product is shipping now in limited supplies.
Website designed by Thasis LLC
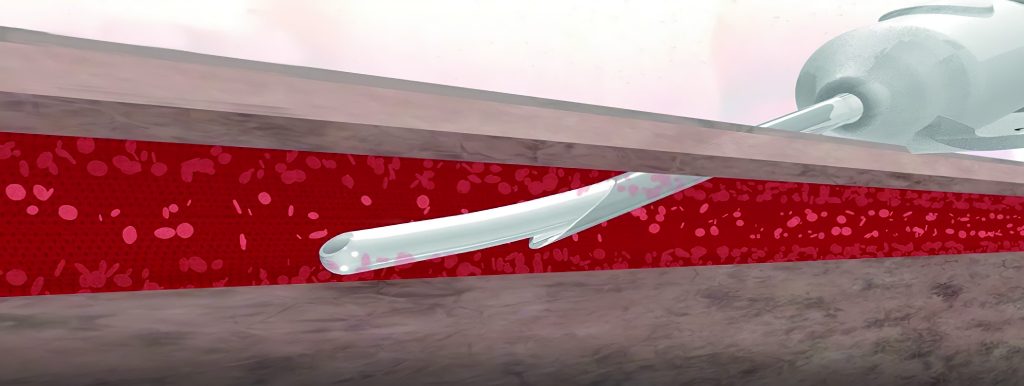
OspreyIV Feature – Contoured Directional Flow
Phlebitis and Infiltration can result from the inflammatory effects of wall shear stress induced by rapid infusions, chemical injuries induced by caustic infusions, or trauma caused by the needle or catheter at the time of initial insertion.
Contoured Directional Flow is designed for atraumatic initial catheter advancement. Once an infusion is initiated, the off-axis opening is intended to deliver fluids away from the delicate vein wall minimizing vessel wall shear stress
* Source references available upon request.
A typical intravenous catheter (over the needle) consists of an inner needle and an outer catheter. Current intravenous catheters on the market have a gap between the outer catheter and the inner needle, the outer catheter also has a tapering or shoulder. This shoulder creates a significant change in push force. When intravenous catheters require greater penetration force, practitioners may push the wall of the blood vessel ineffectively, resulting in unsuccessful catheterization.
In a pilot study involving staff from 2 medical units, 3 surgical units, 2 pediatric units, and 2 intensive care units, data were collected from 371 patients. The mean number of IV insertion attempts was 2.18 (SD = 1.83) Number of attempts ranged from 1 to 14. 27% of patients required three or more insertion attempts.
OspreyIV – Bevel Only Technique
The traditional PIVC insertion sequence includes entering
the vessel with the bevel of the needle and obtaining a blood flash. The angle
is then lowered and further advanced, so the catheter enters the vessel as
well. These manipulations often lead to unsuccessful insertion attempts.
The catheter of the Osprey IV is within the needle. As the
needle enters the vessel, so does the catheter without further device
advancement or re-positioning. Bevel Only Technique is designed to promote high
procedural success rates.
The Occupational Safety and Health Administration (OSHA) estimates that 5.6 million workers in the health care industry and related occupations are at risk of occupational exposure to bloodborne pathogens, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and others. Each year, hospital-based health care workers sustain an estimated 384,000 percutaneous (skin puncture) injuries from needles and other sharp devices or more than 1,000 injuries per day. As many as one-third of all sharps injuries occur during the disposal of the device. *
OspreyIV Feature – Passive Needle Retraction
The Occupational Safety and Health Administration (OSHA) reports as many as one-third of all sharps injuries occur during disposal of the device.
Passive Needle Retraction is designed to eliminate exposure to post-procedure sharps. Once the catheter is fully advanced, the needle automatically retracts into the housing. It is then safely and permanently retained without any sharps to transport for disposal.
* Source references available upon request.
Staphlococcus aureus (S.aureus) is a normally occurring bacteria on and within human skin, within hair follicles, and within sebaceous glands.* It has been identified as one of the most common causes of hospital associated bloodborne infections.*
S.aureus cannot be removed from all layers of the skin prior to a PIV insertion. Most antimicrobial agents effectively eradicate bacteria from the surface but not from the stratum corneum. The rates of eradication from the stratum corneum after surface treatment with 70% ethanol chlorhexidine-ethanol and povidone-iodine were not statistically different from those of the control sites (no surface treatment at all). *
It is understood vascular access devices (PIVCs) may be contaminated by infectious bacteria during insertion by the catheter coming in direct contact with bacterial flora throughout the layers of the skin. This bacterium adheres to the extraluminal surface of catheter, forms large microcolonies, and ultimately detaches into the blood stream to cause infection.3, 7 10-50% of hospital related S. aureus bloodborne infections are associated with PIVCs.2 Infections rates for PIVCs have been reported as high as 1.45%.*
OspreyIV Feature – Skin Avoidance Technology
It is understood vascular access devices such as PIVCs may be contaminated by infectious bacteria during insertion by the catheter coming in direct contact with bacterial flora throughout the layers of the skin.
The Osprey IV deploys the catheter through-the-needle rather than over-the-needle. This unique design is intended to form a physical barrier between the catheter and harmful bacteria on and within the skin and protect the catheter from insertion related contamination.
* Source references available upon request.
A typical intravenous catheter (over the needle) consists of an inner needle and an outer catheter. Current intravenous catheters on the market have a gap between the outer catheter and the inner needle, the outer catheter also has a tapering or shoulder. This shoulder creates a significant change in push force. When intravenous catheters require greater penetration force, practitioners may push the wall of the blood vessel ineffectively, resulting in unsuccessful catheterization.
In a pilot study involving staff from 2 medical units, 3 surgical units, 2 pediatric units, and 2 intensive care units, data were collected from 371 patients. The mean number of IV insertion attempts was 2.18 (SD = 1.83) Number of attempts ranged from 1 to 14. 27% of patients required three or more insertion attempts.
A typical intravenous catheter (over the needle) consists of an inner needle and an outer catheter. Current intravenous catheters on the market have a gap between the outer catheter and the inner needle, the outer catheter also has a tapering or shoulder. This shoulder creates a significant change in push force. When intravenous catheters require greater penetration force, practitioners may push the wall of the blood vessel ineffectively, resulting in unsuccessful catheterization. *
In a pilot study involving staff from 2 medical units, 3 surgical units, 2 pediatric units, and 2 intensive care units, data were collected from 371 patients. The mean number of IV insertion attempts was 2.18 (SD = 1.83) Number of attempts ranged from 1 to 14. 27% of patients required three or more insertion attempts. *
OspreyIV Feature – Bevel Only Technique
The traditional PIVC insertion sequence includes entering
the vessel with the bevel of the needle and obtaining a blood flash. The angle
is then lowered and further advanced, so the catheter enters the vessel as
well. These manipulations often lead to unsuccessful insertion attempts.
The catheter of the Osprey IV is within the needle. As the
needle enters the vessel, so does the catheter without further device
advancement or re-positioning. Bevel Only Technique is designed to promote high
procedural success rates.
* Source references available upon request.
Board of Directors / Advisor
Sharon is a career medical device executive with over 30 years of success across multiple therapeutic areas and geographies. Key achievements in international expansion, business development, strategic repositioning and turnaround, and bringing new technologies through the development process and into commercialization. Broad functional expertise and proven leadership capabilities. Experience: Operating Partner; Altamont Capital, Group Vice President; CR Bard, Vice President; Baxter Healthcare

As PIV/EDC catheters advance during insertion, it is belived that the catheter lays along the bottom of the vien delivering fluid that could cause harm to the areas it touches.

Designed to deliver fluids more efficiently, lowering the potential risk of chemical damage.

Clinicians risk getting stuck by contaminated needles if the don't actively engage the safety mechanisms.

The needle passively retracts into the housing without the need for the clinician to engage as a separate safety mechanism.

Catheter is on the outside of the needle leading to colonized catheters.
The catheter never touches the skin, minimizing the risk of becoming colonized.

Patient are at risk of needle manipulation. Multiple attempts often require two or more catheters and kits and risk vessel damage.

The Needle does not have to penetrate past the initial entry, improving the likelihood of 1st attempt success and reducing the risk of infiltration.